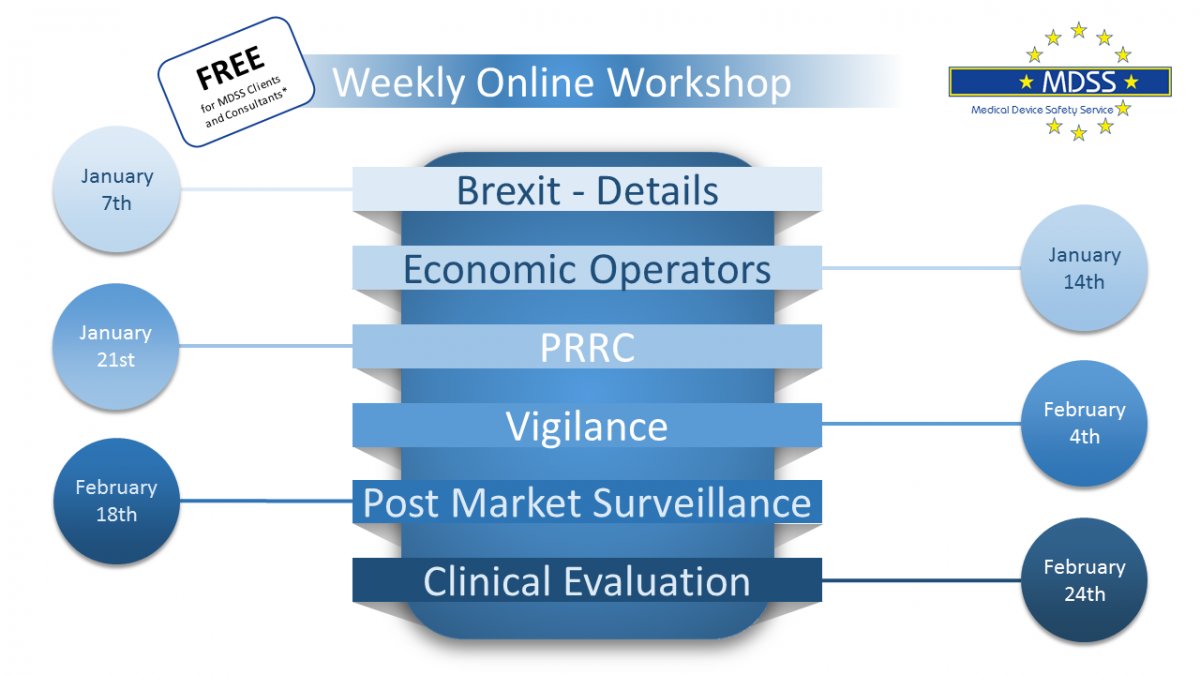
Register now for our Weekly online workshops!
Ask questions. Test your views on the new Regulations!
Ludger Moller will stimulate the dialog and discussion to identify the best scenario for you and your products.
- Since 2000 President Medical Device Safety Service GmbH (MDSS)
Founder MDSS Consulting and ITN Holding GmbH - 1996-2000 Lead Auditor and Expert with leading Notified Body (TÜV Rheinland)
- 1997-2000 Manager of the San Diego TUV office
- 1995-1996 Research and Development of Medical Devices
University degree (Dipl. Engineer), Aachen University of Technology
- Member and Deputy Chair of the European Association of Authorized Representatives (EAAR)
- Member of the European Medical Device Coordination Group (MDCG) and the Commission Working Group on Vigilance
- Member of the Ethics Committee of the Medical Council (Aerztekammer) for Lower Saxony
- Member of the Regulatory Affairs Professional Society (RAPS)
- Founder and President of the RAPS Germany Chapter
| Presenter
Ludger Moeller MDSS President |
Train your whole team with our tailor-made Webinar!
Book this webinars for your company and have your specific needs and internal sensitive topics addressed.
Pick a date of your choice and have all your questions answered!
Workshops will be offered via Zoom




Comments