2022.03.15
Extension of one year for existing “OLD LEGACY PRODUCTS” → Manufacturers must act now to obtain this extra transition!!!!! (see below point 3)
“NEW LEGACY PRODUCTS” with significant additional extension → Manufacturers must act now to issue new DOCs before May 2022 (see below point 4)
Numerous questions concerning the new Regulation (EU) 2022/112, which amends the IVD Regulation (EU)2017/746, are being raised. The amendment intends to ease the transition from the IVDD to the IVDR. It is pertinent for IVD manufacturers to fully understand these new opportunities to stay in business.
The below provides a different approach outlining the new rules with further explanations. Please always refer to the actual regulation to identify the right solution for your devices and your company.
There are four types of products concerning the transition:
1. Low risk class devices under the IVDD (commonly called risk class “other”), which are of risk class A (nonsterile) under the IVDR → NO CHANGES!
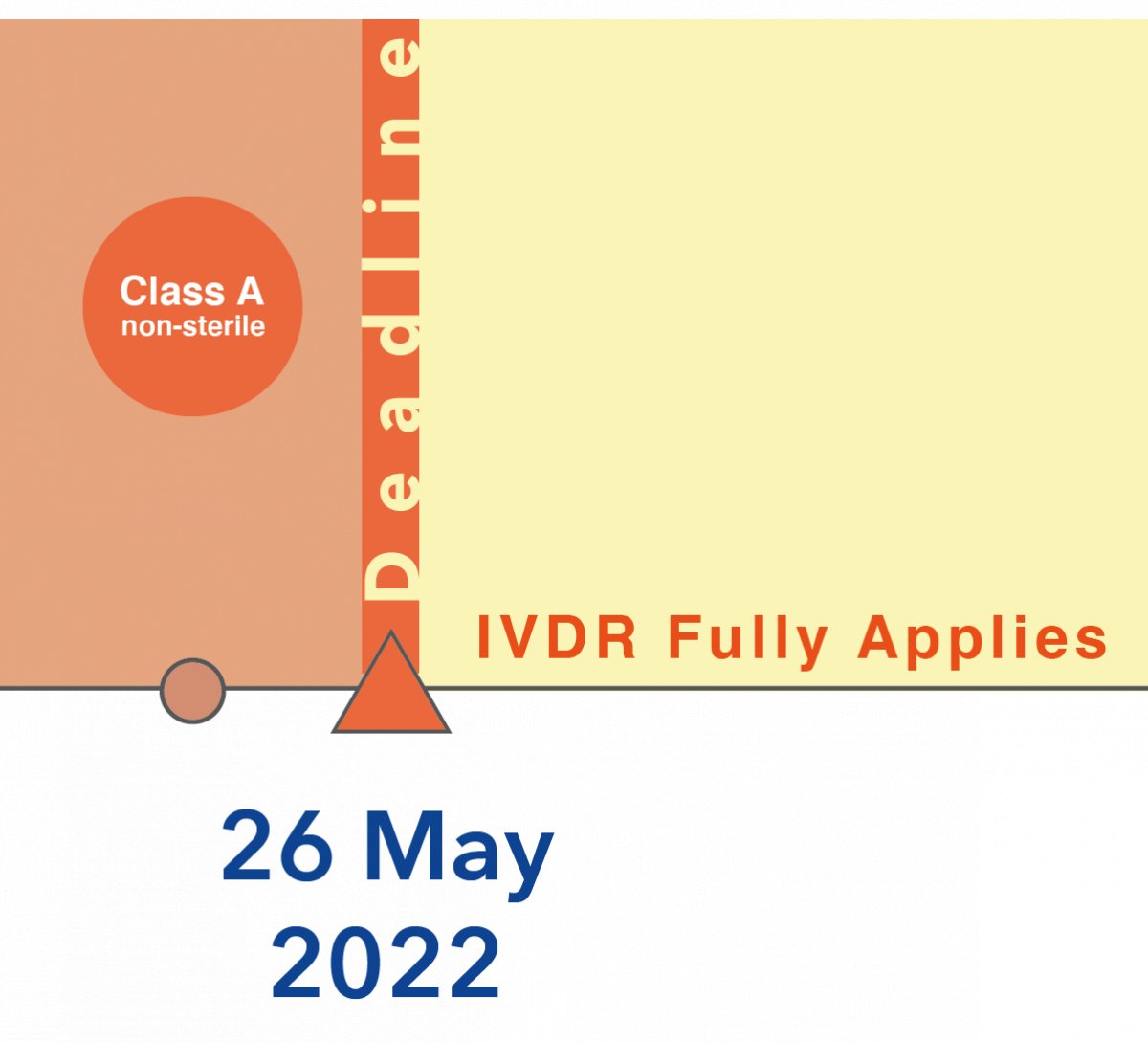 The deadline applies in May 26, 2022 for this risk class. The lowest risk class products still must comply first. The obvious reason is that no notified body is involved and therefore those manufacturers are not affected by the shortage of notified bodies. The manufacturers can implement the requirements themselves and once in full compliance the Declaration of Conformity may be issued according to the IVDR.
The deadline applies in May 26, 2022 for this risk class. The lowest risk class products still must comply first. The obvious reason is that no notified body is involved and therefore those manufacturers are not affected by the shortage of notified bodies. The manufacturers can implement the requirements themselves and once in full compliance the Declaration of Conformity may be issued according to the IVDR.
2. Any Devices newly introduced after the deadline May 26, 2022 independent of the risk class must comply to the IVDR →NO CHANGES!
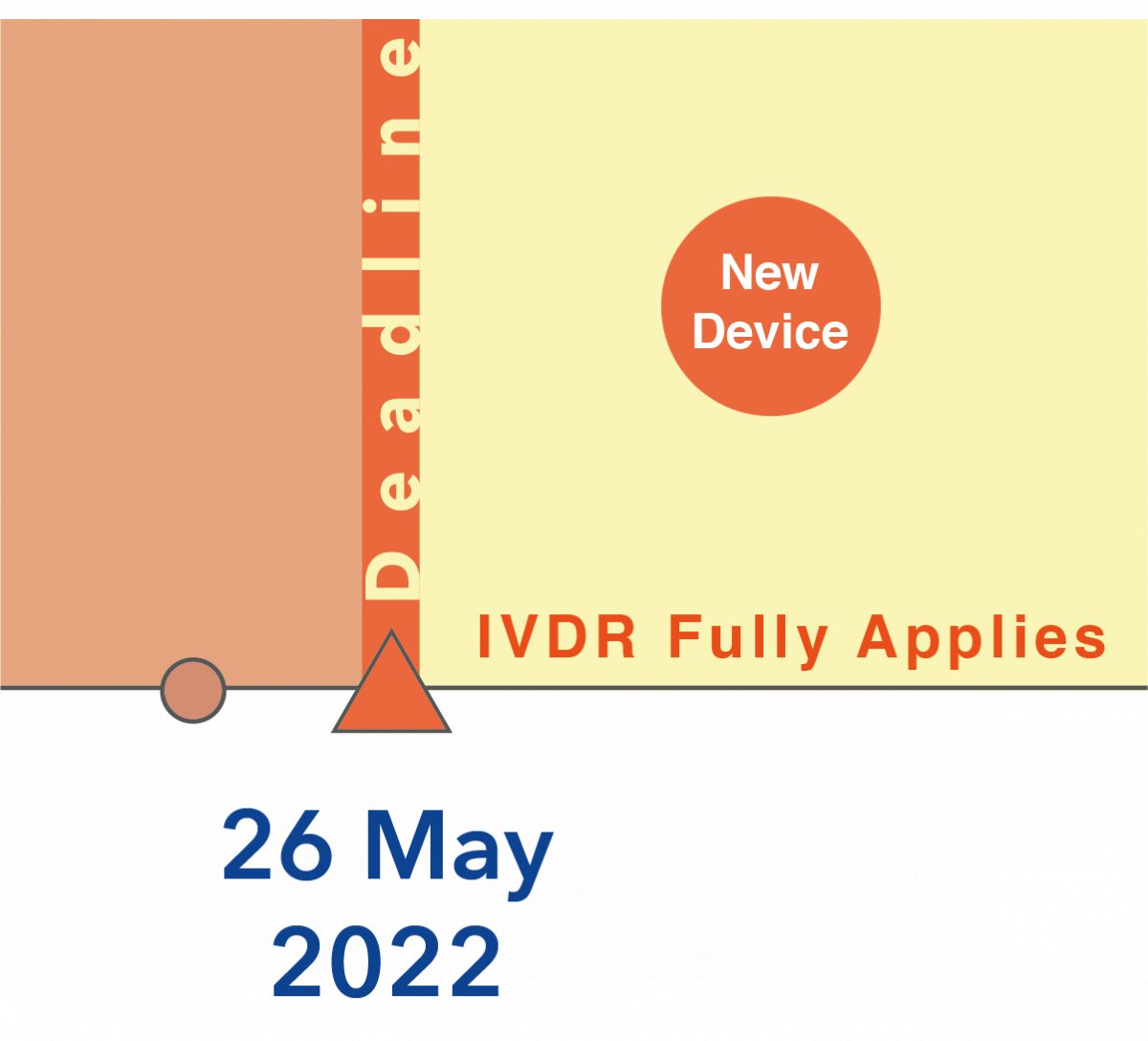
New devices of all risk classes must fully comply with the IVDR upon market introduction after May 26, 2022.
The new extension applies to all LEGACY DEVICES – OLD and NEW! The term legacy device is not as such defined in the regulation. It is commonly being used for those devices, which are allowed to be compliant with the IVDD after the application date of the new regulation (IVDR) under certain specific conditions for a defined period. One condition is that no significant changes concerning the design and the intended purposes are permitted. It triggers immediately the IVDR for a legacy device (see above Number 2 → e.g. a new intended use equals a new device). In essence this is an extra transition for certain established devices. Originally it was foreseen for higher risk class products only, which are under the control of a notified body under the IVDD (let us call them OLD LEGACY DEVICES). An extension of one year is possible.
3. OLD LEGACY DEVICES: devices certified under the IVDD (Annex II and self-testing IVDs) → ONE YEAR EXTENSION POSSIBLE. The new deadline could be May 26, 2025.
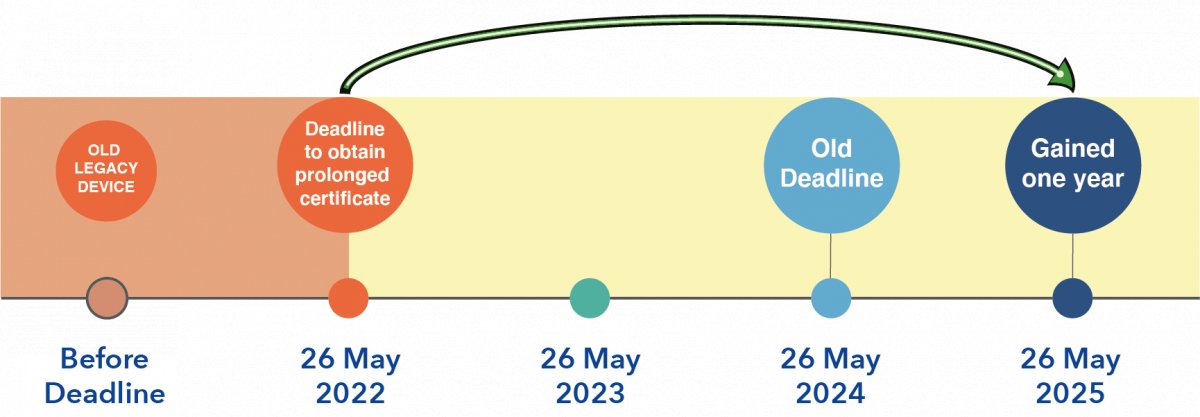 An additional year is being granted to those products if the certificate is still valid. The validity of the certificate must be checked now and manufacturers must receive the updated certificate before May 26, 2022, which outlines the validity until 2025.
An additional year is being granted to those products if the certificate is still valid. The validity of the certificate must be checked now and manufacturers must receive the updated certificate before May 26, 2022, which outlines the validity until 2025.
There are two possibilities:
- The certificate allows for an extension. A certificate validity is limited to five year by the IVDD. To allow for a simple extension your certificate should permit for such an extension. This applies to all certificates issued after May 26, 2020. If this is not the case, then
- A new certificate must be issued. This may cause some other issues.
A new certificate issued must be carefully checked in terms of the scope. For example, a scope which outlines the devices up to the SKU may limit you in terms of introducing minor updates allowed by the IVDR (non-significant changes). Certificates cannot be updated after the deadline. In any case this should be thoroughly discussed with your notified body. THEY MAY PROVIDE FOR OTHER SOLUTIONS.
The additional extension is limited to only one year because those manufacturers already work with a notified body and are familiar with conformity assessment process.
4. NEW LEGACY DEVICES: Devices not previously certified under the IVD (risk class other devices) and which must be certified under the IVDR → BIGGEST CHANGES
A new extension is now applied for a new set of devices (let us call them NEW LEGACY DEVICES), which are of the lowest risk class under the IVDD and are of a higher risk class under the IVDR and because of that they must involve a notified body under the IVDR - they are the NEW LEGACY DEVCIES. There are not enough notified bodies for those devices and therefore the IVDR regulation had to be updated accordingly.
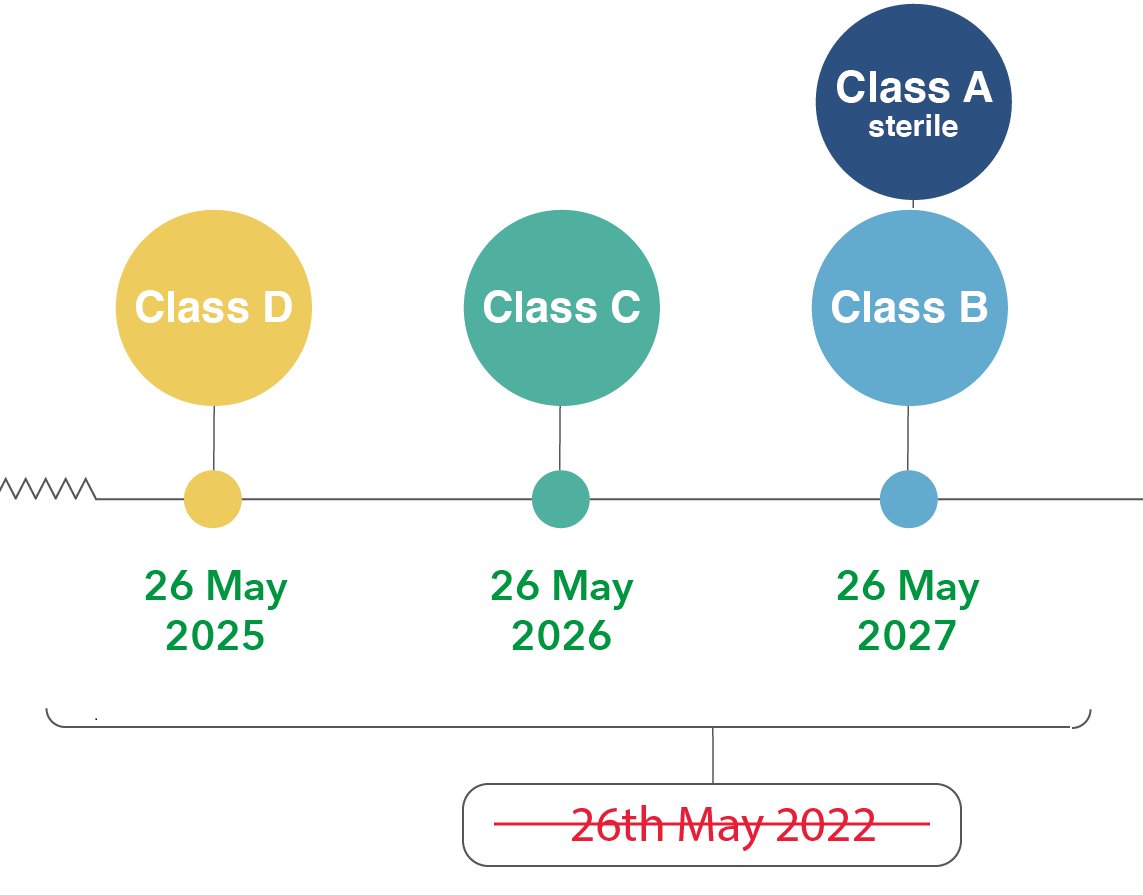
The good NEWS! The amending regulation (2022/112) for the IVDR 2017/746 provides a risk-based approach for a prolonged extension for the NEW LEGACY DEVICES. These devices must comply at a later point depending on their risk potential. This all makes sense.
Manufacturer should act now to review the DOC. Upon the deadline of May 26, 2022 the DOC is fixed and cannot be updated. It would be recommended to review the scope to allow for minor updates of the devices (non-significant).
Please join our Seminar and learn on how to do that!

Comments