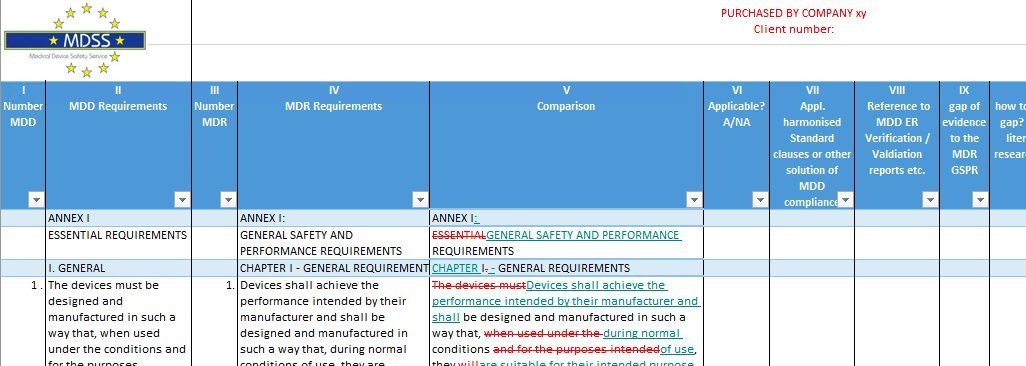
This Excel spreadsheet is designed to support manufacturers making the transition from MDD to MDR.
It is specifically designed for the area of MDD “essential requirements” vs. MDR “general safety and performance Requirements” Annex I.
This checklist once filled out establishes the objective evidence for the MDR compliance. In addition, it still provides the same for the MDD compliance. The checklist is a great tool for the transition to the MDR with your current MDD compliant products. Are you not tired of the Notified Body answer: “What you have done for the MDD essential requirements (ER) will not be sufficient. There is definitely more for you to do with the MDR GSPR."
The checklist will provide an immediate status of the compliance evidence for the MDR GSPR with utilizing the MDD ER information. We expect that for the majority of products - in particular, the lower risk class products compliance may already be quite well established with the current information available for the MDD ER. However, as long as you cannot prove this you may lose it.
The exact differences are laid out therefore; even subtle variances will be caught. Should the current information not be sufficient, this tool will support you to identify and provide the chance to address them quickly. The sooner those gaps are identified, the better.
Once each gap is closed, you will establish full compliance.
This tool is also tremendously important to make use of the “soft transition” with your MDD certificate. This document as well provides for the MDD compliance. It fully supports the MDD certificate for the time being. Meaning you will not be forced to maintain two set of documents. And certainly make it part of your QM System (see article 10 9. (b).
Once all gaps are addressed, then your product compliance is established and you are ready to tackle other MDR aspects with the confidence that your product is safe and performs as intended!
You will receive the document within 24 hours after payment.


Comments